Converting Fallen Leave – Porous Carbon Material
An innovative system of converting fallen tree dried leaves to porous carbon material which could be utilised in producing high tech electronics have been found by researchers in China. Researchers have defined in a study printed in the Journal of Renewable and sustainable energy, on the procedure of converting tree leaves into a system of integrating into electrodes as active resources. Initially the dried leaves are ground into powder and thereafter heated to 220 degrees Celsius for about 12 hours which formed a powder comprised of small carbon microspheres.The carbon microspheres are then said to be preserved with a solution of potassium hydroxide and heated on gradually increasing the temperature in sequences from 450 to 800 degrees Celsius. Due to the chemical treatment it tends to corrode the surface of the carbon microspheres which tends to make it tremendously permeable.
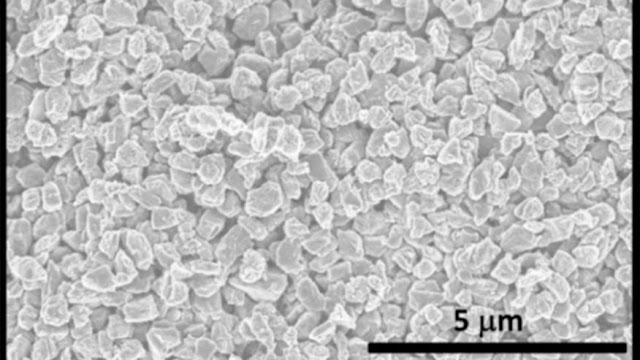
The concluding production which is a black carbon powder is said to have a great surface area owing to the existence of several small holes which tend to have been chemically carved on the surface of the microspheres. The great surface area provides the ultimate produce with unusual electrical properties.
Permeable Microspheres
Led by Hongfang Ma of Qilu University of Technology in Shandong, the detectives followed a succession of standard electrochemical test on the permeable carbon microspheres in order to enumerate their possibility for utilisation in electronic devices.
The current-voltage curves for these materials showed that the element tends to make exceptional capacitor. Additional tests indicated that the materials had in fact been super capacitors having precise capacitances of 367 Fards/gram.
These were said to be over thrice the value seen in some of the graphene super capacitors. Capacitor is said to be an extensively utilised element which tends to store energy on holding a charge on two conductors, which are detached from each other with the support of an insulator.
Super capacitor tend to store 10 to 100 times the energy as an ordinary capacitor and has the tendency of accepting and delivering charges much quicker than a usual rechargeable battery. Hence super capacitive materials have the potentials for an extensive selection of energy storage essential in particular in computer technology as well as hybrid or electric vehicles.
Enhance – Electrochemical Properties
The roadsides of northern China are said to be scattered with deciduous phoenix trees which produce abundant fallen leaves during autumn and these leaves are usually burnt in the colder climate, aggravating the air pollution issue of the country.
The investigators in Shandong, China, had recently found the new system of resolving this issue by means of converting waste biomass into porous carbon materials which could be used in energy storage technology. Besides tree leaves, the team together with the others have also succeeded in changing potato waste, corn straw, pine wood, rice straw as well as other agriculture wastes into carbon electrode materials.
Professor Ma together with her colleagues expects to enhance more on the electrochemical properties of porous carbon materials by augmenting the preparation procedure and enabling fixing or adjustment of the raw materials.